Blog Post 8 (Unit 8)
- Describe how temperature and kinetic energy are related
In most substances, when there is an increase in temperature, either of that which surrounds it, or of the substance itself, the molecules within it begin to move at a quicker rate. Thus also increasing the kinetic energy of the material or substance in question. In the reverse, the lower the temperature becomes, the less kinetic energy becomes present, slowing the molecules, and in the case of water, or other such liquids, would alternate into the state of freezing.
- Explain what factors that affect the rate of reaction and how that rate affects the reaction time.
The rate of reaction determines how complete the reaction is in any given amount of time. Several factors can contribute to this, including temperature, and additional chemical components added to the reaction mixture. Depending on the reaction being performed, the reaction rate would either increase or decrease. This in turn would increase the reaction time along side the decrease of reaction rate, and the decrease of reaction time with the increase of reaction rate.
- What are the 5 different reaction types and how are can you tell the difference?
Click HERE
1. Synthesis - Characterized by the combination of two or more elemental components into a new combined substance.
2. Decomposition - Characterized by the splitting of a combined substance into two or more elemental compounds.
3. Double Replacement - Both components in the reactive substance are replaced/removed, or changed.
4. Single Replacement - A single compound moves places or is exchanged within the reactive substance.
5. Combustion - With the addition of heat, typically involving oxygen within the reaction, typically splits or synthesizes organic compound substances.
- Define, in your own words and describe an endothermic and exothermic reaction.
An Endothermic reaction draws and collects energy from the enviornment surrounding it. The reaction itself therefore ends up with often slightly more energy than the area surronding it.
An Exothermic reaction expels and releases energy from itself into the enviornment surronding it. It then has less energy than the enviornment directly around it, which can be detected and seen easily.
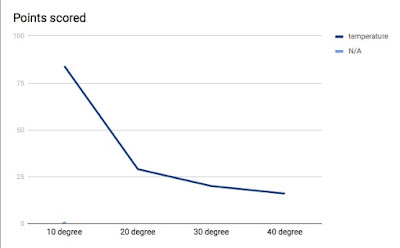
- Include a graph of your time vs. temperature data from Lab 4 (can be computerized, drawn, or a picture)
- For each reaction: a. classify the chemical reaction; b. predict the products, including s, l, g, aq.; c. balance the chemical equation
a) Silver acetate is added to sodium phosphate. AgC2H3O2 (aq) + Na3PO4 (aq) –>
b) Boron metal is burned in air B (s) + O2 (g) –>
c) Hydrochloric acid is added to sodium hydroxide solution. HCl (aq) + NaOH (aq) –>
HCl(aq) + NaOH(aq) --> H2O(l) + NaCl(aq) <-- balanced and completed || Double Replacement Reaction
d) Aluminum metal is added to a solution of copper(II) chloride. Al (s) + CuCl2 (aq) –>
e) Manganese(II) nitrate solution is mixed with sodium hydroxide solution. Mg(NO3)2 (aq) + NaOH (aq) –>
Mg(NO3)2(aq) + 2NaOH(aq) --> Mg(OH)2(s) + 2NaNO3(aq) <-- balanced and completed || Double Replacement Reaction
Mg(NO3)2(aq) + 2NaOH(aq) --> Mg(OH)2(s) + 2NaNO3(aq) <-- balanced and completed || Double Replacement Reaction
f) Hexanol, C6H13OH, is burned in excess oxygen.C6H13OH (g) + O2 (g) –>
C6H13OH(g) + 9O2(g) --> 6CO2(g) + 7H2O(g) <-- balanced and completed || Combustion Reaction
C6H13OH(g) + 9O2(g) --> 6CO2(g) + 7H2O(g) <-- balanced and completed || Combustion Reaction
g) Solid magnesium carbonate is allowed to decompose.MgCO3 (s) –>
MgCO3(s) --> MgO(s) + CO2(g) <-- balanced and completed || Decomposition Reaction
MgCO3(s) --> MgO(s) + CO2(g) <-- balanced and completed || Decomposition Reaction
h) Lithium peroxide is heated. Li2O2 (aq) –>
2Li2O2(aq) + 2H2O(l) --> 4LiOH(aq) + O2(g) <-- balanced and completed || Redox Reaction
2Li2O2(aq) + 2H2O(l) --> 4LiOH(aq) + O2(g) <-- balanced and completed || Redox Reaction
i) Potassium oxide is added to water. K2O (aq) + H2O (l) –>
K2O(aq) + H2O(l) --> 2KOH (aq) <-- balanced and completed || Redox Reaction
K2O(aq) + H2O(l) --> 2KOH (aq) <-- balanced and completed || Redox Reaction
j) Solid sodium cyanide is added to water. NaCN (aq) + H2O (l) –>
NaCN(aq) + H2O(l) --> NaOH(aq) + HCN(s) <-- balanced and completed || Redox Reaction
NaCN(aq) + H2O(l) --> NaOH(aq) + HCN(s) <-- balanced and completed || Redox Reaction