Blog Post 6 (Unit 6)
Atomic structure of gold particulate taken using an electron microscope.
- Define, compare, and contrast between ionic, metallic, and covalent bonding
Ionic bonding occurs when two ion forms exchange electrons between them, freely. This creates both positively and negatively charged particles as the end result.
Covalent bonding is the bonded "sharing" or electrons between two atomic particles, where the electrons remain held by both structures.
Metallic bonding occurs between two metals, where electrons are used freely between the separate forms. Charged ionic particles may split off from one, and go towards the other.
- Draw a Lewis dot structure and include a picture of your drawing of NCl3
- What is the molecular geometry of NCl3 and explain using VSEPR theory why that is the shape.
NCl3 is a Trigonal Pyramidal elemental shape. The molecule has four pairs of electrons, meaning the most stable shape, with least resistance for the electron pairs is tetrahedral. Due to the repulsion properties of the differently placed electrons, the lone pair, or unbonded set of electrons is closer to the center of the atomic structure. The non bonded pair of NCl3 is placed on the top of the structure, requiring more room than the other pairs of electrons within the structure.
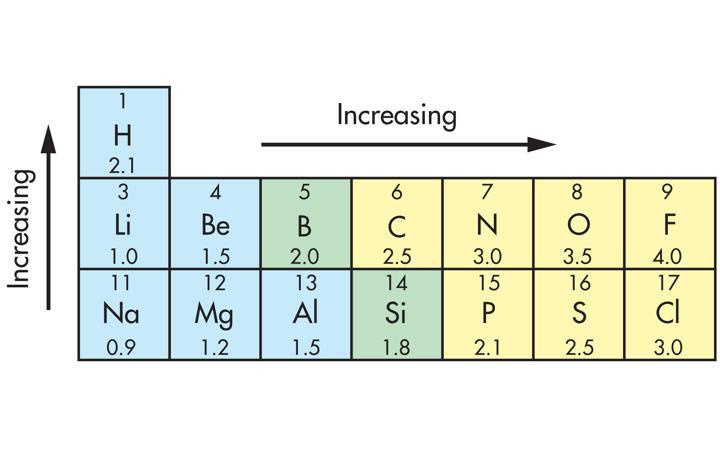
- How can the electronegativity of atoms be used to predict the types of bonds in molecules? Help
Electronegativity, the calculated measure of an atomic structure's attraction of electrons. The nearer the atom's valence electron count is to a complete octet (eight electrons present) the greater their electronegativity or electron attraction becomes. The type of predicted bond formed between the two atoms depends upon the difference between the two's electronegativity. If the difference is greater, the bond will become ionic, with one atom removing electrons from the other. If it is a small difference between the atomic structures, the bond will become non-polar covalent, with the electrons sharing between them. If the difference is somewhere between the two, or a moderate distance apart, the bond will be polar covalent; still shared, but drawn towards the increasingly electronegative one.
- How are empirical and molecular formulas distinguished?
Molecular formulas are direct, exact representations of an atomic model, containing the exact number of atoms within the structure. This is shown often as a ratio-like set of numbers that represent the overall structure of the atom. As a ratio, it can be reduced, and the most highly simplified version of this is named the empirical formula. Empirical formulas and Molecular formulas can both be found using the molar mass shown in the periodic table.
- Explain how ionic compounds are named?
Ionic compounds are composed of multiple atomic structures in a way that the overall charge is zero, making them neutral by nature. To begin with, the formula of the compound is written out. To name ionic compounds, the cation or name of the element is named first, followed then by the name of the anion.
- What are the rules for naming binary molecular compounds (covalent)?
The binary molecular compounds are named using numerical prefixes depending on how many of each atom are present in the compound. The first of the element uses its element name, as usual, the second uses its root both in combination with the atomic greek prefixes.
- How do scientists name acids?
Acids are identified by a cation of Hydrogen. They are named through looking at their molecular suffixes, which determines the type of acid that it is. If the Acid's ending is ide, the name/type of acid becomes hydro___ic acid, likewise, if it ends in ate, it becomes ___ic acid, and the suffix ite is ___ous acid.

I love your blog and all the photographs and how organized it is, on my computer one of the photos won't show up, but other than that the blog is good
ReplyDelete