Blog Post 7 (Unit 7)
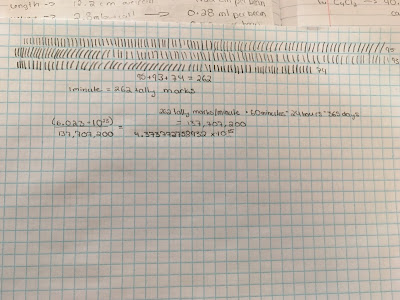
- Make as many tally marks as you can in 1 minute. How many years would it take you to make a mole of tally marks if you stayed at the same pace without stopping? (Show your work).
- What is the molar mass of potassium permanganate? (Show your work).
source: click
K = 39.0983amu
Mn = 54.938
O = 15.999 4(15.999) = 63.996
K+Mn+O4 = 39.0983amu + 54.938amu + 63.996amu = 158.0323 g/mol
Mn = 54.938
O = 15.999 4(15.999) = 63.996
K+Mn+O4 = 39.0983amu + 54.938amu + 63.996amu = 158.0323 g/mol
- Calculate the percent composition of each element in silver sulfide. (show your work).
Ag2S
Ag = 107.8682 2(107.8682) = 215.7364amu
S = 32.065amu --> 215.7364amu + 32.065amu = 247.8014 g/mol
Ag% --> 215.7364 g/mol // 247.8014 g/mol = 0.8706 x 100 = 87.0602%
S% --> 32.065 g/mol // 247.8014 g/mol = 0.1294 x 100 = 12.9398%
Ag = 107.8682 2(107.8682) = 215.7364amu
S = 32.065amu --> 215.7364amu + 32.065amu = 247.8014 g/mol
Ag% --> 215.7364 g/mol // 247.8014 g/mol = 0.8706 x 100 = 87.0602%
S% --> 32.065 g/mol // 247.8014 g/mol = 0.1294 x 100 = 12.9398%
- Honors Chemistry: Calculate the empirical formula if a compound is 17.6% Na, 39.7% Cr, and 42.7% O. (Show your work). Regular chemistry: Calculate the percent composition of each element of iron (III) chloride.
Na - 17.6% --> 17.6g/22.99g = 0.7665 -----> 0.7665/0.7636 = 1 2(1) - (multiplied by 2): 2
Cr - 39.7% --> 39.7g/51.99g = 0.7636 -----> 0.7636/0.7636 = 1 2(1) - (multiplied by 2): 2
O - 42.7% --> 42.7g/15.999g = 2.6689 -----> 2.6689/0.7636 = 3.5 2(3.5) - (multiplied by 2): 7
Compound Imperical Formula = Na2Cr2O7
Cr - 39.7% --> 39.7g/51.99g = 0.7636 -----> 0.7636/0.7636 = 1 2(1) - (multiplied by 2): 2
O - 42.7% --> 42.7g/15.999g = 2.6689 -----> 2.6689/0.7636 = 3.5 2(3.5) - (multiplied by 2): 7
Compound Imperical Formula = Na2Cr2O7
- Why did you have to heat the hydrate sample twice in the lab? What evidence would have indicated you inadvertently overheated the hydrate?
By sufficiently heating the sample twice, one could ensure that there was no remaining water left in the sample. As there were two collected points of data from the post-evaporation sample, if there was no change between the first and second heating, it could be determined that there was no remaining water in the sample that could be detected. Overheating results in the production of Copper (II) Oxide, which would affect the amount of water shown to be present.
- (This question is worth 30 points, remember to show your work) A student is given a sample of green nickel sulfate hydrate. She weighs the sample in a clean try test tube and obtains a mass of 22.326 g. Earlier she had found that the test tube weighted 21.244 g. She then heats the test tube to drive off the water of hydration. She then lets the test tube to drive off the water of hydration. She lets the test tube cool, and finds it has a lower mass; the test tube and contents then weigh 21.840 g. In the process the sample was converted to yellow anhydrous NiSO4. Fill in the missing parts of the chart:
source: click
a. Mass of Empty Test tube __21.244g___
b. Mass of test tube & NiSO4 * ??? H2O ___22.326g____
c. Mass of test tube and NiSO4 (after last heating) ___21.840g____
d. Mass of NiSO4 ____22.326g - 21.244g = 1.082g <-- with water included
21.840g - 21.244g = 0.596g <-- without water included____
21.840g - 21.244g = 0.596g <-- without water included____
e. Mass of H2O _____1.082g - 0.596g = 0.486g_______________
f. % NiSO4 in hydrate _mass = 0.596/1.082g * 100 = 55.083%_________________
g. % H2O in hydrate _mass = 0.486g/1.082 * 100 = 44.917%_________________
Molar mass of NiSO4 = 154.8 g h. Molar mass of H2O _= ([H]1.008amu)*(2) + [O]15.999amu = 18.015g/mol__________________
i. Moles of NiSO4 __154.8g/mol <-- One mol of NiSO4 --> 0.596g/154.8g/mol = 0.0038501 Moles of NiSO4___________________
j. Moles of H2O _18.015g/mol <-- One mol of H2O --> 0.486g/18.015g/mol = 0.0269775 Moles of H2O___
k. Mole ratio of moles of NiSO4 to moles of H20 _0.00385:0.0270 | 0.00385/ --> 0.00385:0.0270 <-- /0.00385 = 1:7 (NiSO4:H2O)_________________________
l. formula of the hydrate _1 CuSO4 * _7 H2O <-- Found using the ratio.

-sulfate-photo.jpg)